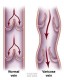
Coronary heart disease (CHD)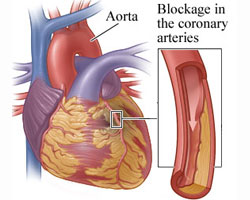 is a disease in which plaque, through a process called atherosclerosis, accumulates inside the arteries that supply oxygen-rich blood to the heart. The plaque, over many years can slowly narrow the arteries supplying the heart or break off and travel from the diseased artery and completely block blood flow elsewhere. Another threatening scenario can occur when a blood clot forms at the site where the plaque breaks off the diseased artery. Similarly to plaque formation, the blood clot can narrow and completely obstruct blood flow as it increases in size.
is a disease in which plaque, through a process called atherosclerosis, accumulates inside the arteries that supply oxygen-rich blood to the heart. The plaque, over many years can slowly narrow the arteries supplying the heart or break off and travel from the diseased artery and completely block blood flow elsewhere. Another threatening scenario can occur when a blood clot forms at the site where the plaque breaks off the diseased artery. Similarly to plaque formation, the blood clot can narrow and completely obstruct blood flow as it increases in size.
Cardiac tissue is made up mostly of cardiac muscle. Smooth muscle, in addition to nervous, connective and endothelial tissue forms the vessels, valves and electrical conduction system supporting the overall function of cardiac muscle. The contracting and relaxing cardiac muscle is responsible for pumping blood throughout the body and has poor to no regenerative capacity once damaged.
Scar tissue replaces damaged cardiac muscle and its original function is impaired or lost. A myocardial infarction (MI), also known as a heart attack, is a complication of CHD resulting in the death of cardiac tissue. Sudden death from a MI may occur if a significant or specific portion of heart is damaged. Further complications can arise in those who survive a MI secondary to the impairment of either the pumping action of the cardiac muscles, the valves that open and close with each heart beat or the electric conduction which produces the rate and necessary rhythm to adequately supply the entire body with oxygen-rich blood.
Significant advances in medical and interventional therapies currently allow patients who suffer from a MI to live longer than ever before. Unfortunately, many of these individuals will gradually develop heart failure in their later life due to irreversible loss of cardiac muscle cells. There are no current treatments available that can repair damaged cardiac tissue once it has occurred. This article will discuss a promising and potential new treatment using stem cells. This technique is currently being studied and gives hope for the possibility of repairing damaged cardiac tissue and restoring the function lost to the complications of CHD.
Stem Cells
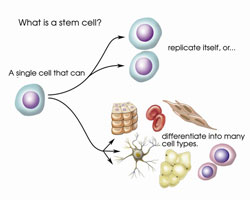 Stem cells are undifferentiated cells. These special cells can differentiate into all other types of cells with specialized functions in the human body. No other cell in the body has this ability. Stem cells occur naturally in the body and under the right conditions, can divide to form more cells called daughter cells which in turn can become new stem cells or become new blood, brain, bone and heart cells.
Stem cells are undifferentiated cells. These special cells can differentiate into all other types of cells with specialized functions in the human body. No other cell in the body has this ability. Stem cells occur naturally in the body and under the right conditions, can divide to form more cells called daughter cells which in turn can become new stem cells or become new blood, brain, bone and heart cells.
Researchers have discovered several sources of stem cells including embryos, amniotic fluid, umbilical cord blood and adult cells. Embryonic stem cells come from embryos that are just days old. These cells form after an egg is fertilized by sperm in vitro at a laboratory. The process of using cells from fertilized eggs has led to controversy from right-to-life advocates. Researchers are permitted to use such fertilized eggs for stem cells only after the requests of the patients at each in vitro fertilization clinic have been met.
Also, amniotic fluid that surrounds and the umbilical cord blood that nourishes the growing fetus contains stems cells. Amniotic stems cells can be obtained from a pregnant woman through a procedure called amniocentesis during the prenatal period. Umbilical cord blood is the blood that remains in the attached umbilical cord which is extracted after the labor and delivery of a child.
Adult stem cells are found in small numbers in most tissues. However, adult stem cells are more limited than the other sources in their ability to differentiate into the various specialized cells of the body. Additionally, scientists have successfully found a way to transform regular adult cells into stem cells by altering the cell's genes.
This may allow researchers to use these reprogrammed adult cells instead of embryonic stem cells. Also, since such stem cells could be self-donated, it has been proposed to be a way to prevent possible immune system rejection of stem cells donated from others. However, more research is needed to understand how altering genetics may impact the differentiated cells long term.
A novel approach – Restoring function
Results from a clinical trial performed at the Cedars-Sinai Heart Institute show that an infusion of cardiac stem cells harvested from the same patients who were treated, helped those patient's damaged hearts regrow healthy muscle. The harvested cardiac stem cells were grown in a laboratory prior to being inserted at the sites of injury caused by the MI. The MI patients who received stem cell treatment demonstrated a significant reduction in the size of the scar left on the cardiac muscle 1.
Researchers from the University of Louisville have completed a phase 1 trial, which screens for safety, to see if cardiac stem cells improved post-MI dysfunction similarly in humans as it was shown to do so in animals. The initial results suggest that infusing cardiac stem cells derived from the same individual was effective in improving function and reducing the scar size in patients with heart failure after a MI. These encouraging results warranted researchers to begin phase 2 trials, which establishes the efficacy of the treatment, usually against a placebo 2.
A clinical trial conducted by researchers at King's College London on rodents proved that cardiac muscle contains stem cells with the capacity to regenerate. They found that destroying such stem cells suppressed the ability of the heart to regenerate and recover function. However, when laboratory grown cardiac stem cells were reinjected into the damaged cardiac muscle, the cells migrated to the damaged sites. These findings could lead to less invasive treatments or possibly preventative measures targeting the activity of the heart's own stem cells 3.
Finally, decades of evidenced-based medicine could be transformed by the utilization of stem cells in the treatment of CHD. The current mainstay of preserving cardiac function after damage would be replaced by stem cell therapy geared towards returning the heart to its original function. The magnitude and impact of such therapy would save countless lives, improve the quality of life of millions of individuals and potentially save billions of dollars annually spent on the existing treatments of CHD and the complications related to it 4.
- Makkar RR, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomized phase 1 trial. Lancet. 2012 Mar; 379 (9819): 895 – 904.
- Bolli R, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomized phase 1 trial. Lancet. 2011 Nov 26; 378 (9806): 1847-57.
- Ellison GM, et al. Adult c-kit pos Cardiac Stem Cells Are Necessary and Sufficient for Functional Cardiac Regeneration and Repair. Cell. 2013 Aug; 154 (4): 827–842.
- Liu JL, et al. The economic burden of coronary heart disease in the UK. Heart 2002; 88: 597-603.